|
FRAGILE X SYNDROME |
Fragile X syndrome is the most common cause of inherited mental retardation, seen in approximately one in 1,200 males and one in 2,500 females. Males with fragile X syndrome usually have mental retardation and often exhibit characteristic physical features and behavior [Hagerman and Silverman, 1991; Warren and Nelson, 1994]. Affected females exhibit a similar, but usually less severe phenotype.
Although it is thought to be an X-linked recessive trait with variable expression and incomplete penetrance, 30% of all carrier women are also affected. The syndrome is called “fragile-X” because there exists a fragile site or gap at the end of the long arm of the X-chromosome in lymphocytes of affected patients when grown in a folate deficient medium.
Carrier females typically have a 30 to 40% chance of giving birth to a retarded male and a 15 to 20% chance of having a retarded female. Further, there frequently exists a maternal family history for a relative with mental retardation or developmental and learning disabilities. Most studies have dealt with recognition of this syndrome in older children and young adults, but many of the physical features, behavioral characteristics, and family history features are apparent earlier.
CLINICAL FINDINGS |
Developmental delay, speech delay, short attention span or hyperactivity, mouthing of objects persisting at an age beyond expected, difficulty in disciplining the child, frequent temper tantrums, autistic-like behaviors such as rocking, talking to oneself, spinning, unusual hand movements, difficulty with transitions, preference for being alone, echolalia, poor eye contact; poor motor coordination; history of vomiting, spitting up or colic during infancy; history of frequent otitis media; self-abusive behavior; hand flapping; drooling persisting beyond expected; hypotonia; increase fighting with others; pica; hand/thumb sucking.
While older children (8-12 years) are more likely to display the classic physical features of fragile X syndrome (long face with a prominent jaw, large prominent ears, and post-pubertal macroorchidism, patients as young as 2 or 3 years have been noted to exhibit the following physical findings: Long and/or wide and/or protruding ears; prominent jaw or long face; high arched palate; flattened nasal bridge; microcephaly or relative macrocephaly; apparent hypertelorism; epicanthal folds; simian creases of palms, vertical creases of soles; long philtrum; hemangioma; hyperextensible joints; antimongoloidal slant; clinical impression of macroorchidism, prominent forehead.
PHYSICAL FINDS / ULTRASOUND |
Long, wide, or protuding ears, a long face, flat nasal bridge, and a high arched palate, lax joints and (in males) enlarged testes

GENETICS |
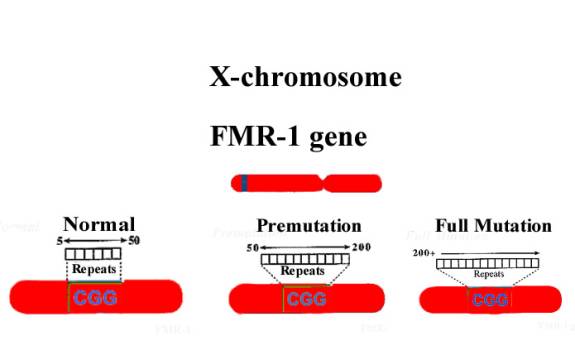
Fragile X syndrome is caused by mutation of the FMR-1 gene on the X chromosome. The FMR-1 gene contains a sequence that consists of a variable number of repeats of the trinucleotide CGG. This sequence occurs in a part of the gene that is transcribed but is not translated into protein. The normal number of CGG repeats varies between 5 and about 50 (average around 30). Individuals with fragile X syndrome typically have more than 200 of these repeats, a condition known as a full mutation (FM). The full mutation prevents transcription of the FMR-1 gene, so that none of its protein product is made. Males have only one X chromosome, so if they carry a FM they are always affected. Females have two X chromosomes and the result of a FM in one chromosome can be very variable: about 50% of such females show some symptoms of the syndrome and 20% are severely affected.
The unaffected mothers of fragile X individuals are invariably found to have an FMR-1 gene containing between 50 and 199 CGG repeats; this intermediate number is known as a premutation (PM).
The chances of a PM in a mother expanding to a FM in her child have been estimated at about 10% in the general population and about 60-80% in known fragile X families. In contrast to the potential instability of a PM transmitted from the mother, a PM transmitted from the father does not expand to a FM in his daughters. This means that all the children of a male with a PM are unaffected (his sons do not inherit his X chromosome), but because all of his daughters inherit the PM they are at risk of having a child with a FM.
In 1991, the fragile X gene (FMR1) was characterized and found to contain a tandemly repeated trinucleotide sequence (COG) near its 5' end. The mutation responsible for fragile X syndrome involves expansion of this repeat segment. The number of CGG repeats in the FMR1 genes of the normal population varies from six to approximately 50. There are two main categories of mutation, premutations of approximately 50 to 200 repeats and full mutations of more than approximately 200 repeats. There is no clear boundary between the upper limit of normal and the lower limit of the premutation range. For this reason, alleles with approximately 45-55 copies of the repeat are said to be in the "grey zone." Some alleles in this size range are unstable and expand from generation to generation, while others are stably inherited. A premutation is susceptible to expansion after passage through a female meiosis. The larger the size of a woman's premutation, the more the risk of expansion to a full mutation in her offspring.
REFERENCES |
1. Barnicoat, A. Screening for fragile X syndrome: a model for genetic disorders? (Editorial) BMJ 1997;315: 1174-1175.
2. De Vries, B.B.A., Halley, D.J.J., Oostra, B.A. and Miermeijer, M.F. The fragile X syndrome. J. Med Genet. 1998; 35: 579-589.
3. Turner, G., Robinson, H., Wake, Laing, S. and Partington, M. Case finding for the fragile X syndrome and its consequences. BMJ 1997; 315:1223-1226.
4. Hagerman RJ, Silverman AC. "Fragile X syndrome: Diagnosis, Treatment, and Research, 1991;" Baltimore: Johns Hopkins University Press.
5.
Warren ST, Nelson DL. Advances in molecular analysis of
fragile X syndrome. JAMA 1994; 271:536-542.