|
THE PATHOLOGY OF TROPHOBLASTIC INVASION |
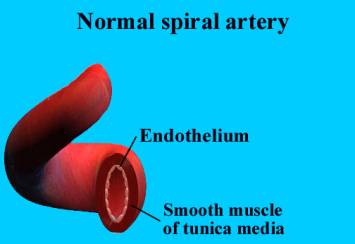
The main branch of each uterine
artery enters the uterus just above the cervix and ascends along the lateral
part of its wall. These peripheral branches give rise to arcuate arteries that
derive their name from the arching pattern in the uterus. The arcuate arteries
give off multiple branches of penetrating vessels called radial arteries. As
the radial arteries approach the uterine cavity they become spiral arteries
THE NON PREGNANT STATE
|
In the non-pregnant state the uterine vessels carry less than 1% of the maternal cardiac output (1). This is not surprising in light of the fact that a nonpregnant women only needs to maintain a uterus that weighs 50 g. At term these same vessels must support a uterus, placenta and fetus that can weigh up to 5,000 g.
Poiseuille’s law of fluid flow in a cylinder reveals that flow is proportional to the radius to the fourth power (2). Comparison of vessels in the nonpregnant uterus to those at term reveals that these vessels can increase their radii by as much as ten fold. This translates into an increase in blood flow by a factor of 10,000. Clearly the ability of uterine vessels to vary in diameter is a great advantage.
INVASIVE TROPHOBLASTS, DECIDUALIZATION AND
MENSTRUATION
|
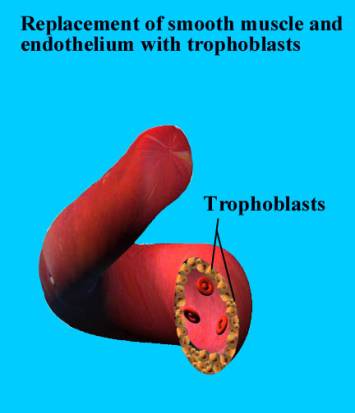
Invasive trophoblasts are the key to the modulation of the state of the uterine vessels (3). These unique cells leave the placenta, penetrate the endometrium and upper layers of the myometrium, selectively permeate the uterine spiral arteries and modify these vessels to yield widened, low resistance vascular channels that carry the markedly increased maternal blood flow to the placenta. This represents a very delicate balancing of conflicting biological needs between the mother and fetus. The fetus, on the one hand, requires its invasive trophoblasts to aggressively penetrate the mother’s uterus in search of vessels to modify. The mother, on the other hand, must protect herself from the invasive trophoblasts, lest they completely penetrate her uterus, causing her to hemorrhage and bleed to death.
FORMATION OF INVASIVE TROPHOBLASTS
|
Two types of trophoblasts have been traditionally described: the cytotrophoblast and the syncytiotrophoblast. The cytotrophoblast; the endocrinologically active villous syncytiotrophoblast; the junctional trophoblast that attaches the anchoring villi to the maternal decidua at Nitabuch’s layer; and the invasive intermediate trophoblast that migrates into the decidua, the myometrium and finally the spiral arteries of the uterus (4).
Cytotrophoblast:
-
The stem cell of the
placenta.
- Gives rise
to the differentiated forms of trophoblasts.
-
Within
the chorionic villi, cytotrophoblasts fuse to form the overlying
syncytiotrophoblast.
- The villous syncytiotrophoblast makes the majority of the placental hormones, the most studied being hCG.
- At the point where chorionic villi make contact with external extracellular matrix, a population of trophoblasts proliferates from the cytotrophoblast layer to form the second type of trophoblast—the junctional trophoblast. The junctional trophoblasts make a unique fibronectin—trophouteronectin (TUN)—that appears to mediate the attachment of the placenta to the uterus. TGFß, and more recently, leukemia inhibitory factor (LIF), have been shown to down regulate hCG synthesis and upregulate TUN secretion.
- A third type of trophoblast differentiates towards an invasive phenotype and leaves the placenta entirely. The invasive intermediate trophoblast, in addition to making human placental lactogen, also make urokinase-type plasminogen activator (u-PA) and type 1 plasminogen activator inhibitor (PAI-1)..
Decidualization
In order to protect the mother from the onslaught of invasive trophoblasts migrating towards the uterine spiral arteries, the endometrial stroma transforms itself into a dense cellular matrix known as the decidua (5). The decidua impedes the movement of invasive trophoblasts both by forming a physical barrier to cell penetration and by generating a local cytokine milieu that promotes trophoblast attachment rather than invasion.(6-10). The fate of the invasive trophoblasts is, in part, likely the result of the balancing of the invasive promoting proteases made by the trophoblasts and the inhibitors of invasion made by the decidua(11-14).
The first signs of the decidualization reaction can be seen as early as day 23 (10 days after the peak of the LH surge) of the normal menstrual cycle when the spiral arteries of the endometrium first become prominent (15). Over the next few days the stromal cells surrounding the spiral arteries become increasingly eosinophilic and enlarged as the differentiating effects of progesterone transforms these cells into predecidual cells (16). The progressive decidualization of the endometrial stroma in the later part of the menstrual cycle prepares the uterine lining for the presence of the invasive trophoblasts, but simultaneously closes the door to implantation(17,18). While the state of the endometrium in the later part of the cycle is ideal to protect the mother from the invasive trophoblasts in the event of a pregnancy, it is entirely unsuited for implantation.
Menstruation
Menstruation, the breakdown and sloughing of the endometrial lining at the end of a hormonally driven cycle, and is a mechanism by which the endometrium reestablishes a receptive phase following a cycle of nonconception.
Trophoblast Invasion
The morphologic aspects of human trophoblast invasion has been examined in great detail over the last twenty years (12, 19-28).
Examination of monkey implantation sites has revealed that trophoblasts begin to migrate down into the maternal spiral arteries as early as 10 days after fertilization, and at 14 days, many of the spiral arteries beneath the conceptus are totally occluded (29). The specificity of this vascular interaction is revealed by the fact that no such invasion takes place in the veins.
Hustin and Schaaps, using anatomic and ultrasonographic approaches, suggested that there is in fact trophoblast plugging of the maternal spiral arteries and a coincident decrease in maternal perfusion of intervillous space up until 12 weeks of gestation (30).
Rodeshch et al. (31). then hypothesized that it is critical that maternal blood flow to the embryo be limited very early in gestation to protect the conceptus from excessively high oxygen levels during critical, early stages of differentiation.
This concept was supported by Coppens et al. (32) serial ultrasounds on normal pregnant women between 8 and 14 weeks showed no uteroplacental blood flow in the first trimester but a significant increase at approximately 12 weeks, which reached maximal levels at 14 weeks.
Burton
et al. critically examined the Boyd Collection, 12 early-pregnancy hysterectomy
specimens ranging from 43 to 130 days of gestation housed in the Department of
Anatomy at the University of Cambridge, and showed that there was significant
blockage of the maternal spiral arterioles by trophoblasts at points of contact
with the intervillous space between 6 and 8 weeks but that this blockage was
gradually eliminated between 8 and 12 weeks of gestation (33).
If we accept trophoblast plugging and the first trimester low-flow concept, one question remains: how are the first trimester embryo’s nutritional needs met? Hustin and Schaaps suggested that the intervillous space is bathed by an acellular fluid that could be plasma filtered by the trophoblastic shell.
Burton and colleagues have offered
another possibility. By examining multiple human implantation sites preserved
in the Boyd collection (33), these investigators noted that below openings to
the intervillous spaces there were dilated endometrial glands. It is well known
that the endometrial glands of early pregnancy are characterized by
hypersecretion (34).
Burton and colleagues have suggested that secretions from the hypersecretory endometrial glands contribute nutrients to the embryo in the first trimester. Concomitant with endovascular plugging of the maternal spiral arteries, the process—of trophoblast penetration of the maternal spiral arteries and their conversion to low-resistance channels—begins
The first trimester low-flow concept has not been universally accepted (35-37).
Jauniaux et al. (38) report the direct documentation of a significant increase in placental intervillous oxygen tension, and hence maternal perfusion of the placenta, between 8 and 12 weeks of gestation. This group has also reported in this article that coincident with this increased perfusion and oxygen tension within the placenta between 8 and 12 weeks there is a corresponding increase in anti-oxidant systems, including catalase, glutathione peroxidase and superoxide dismutase, presumably to counteract the oxidative stress of the increased intervillous perfusion and oxygen tension.
UTEROPLACENTAL BLOOD FLOW IN PEGNANCY
|
The action of the invasive trophoblasts on the maternal spiral arteries leads to a very low resistance uteroplacental circulation which facilitates the marked increase in blood flow seen in these vessels at term. Utilizing a variety of techniques, many groups have estimated the amount of blood flow into the gravid uterus (39-43). This work has demonstrated that at term a women’s total blood volume increases by about 40% compared to her nonpregnant state (44). Concomitantly, her cardiac output rises 30-35% and the total uteroplacental blood flow increases to about 25% of her total cardiac output (45,46). Direct measurements of uterine blood flow in the nonpregnant state have shown a combined uterine artery flow in the follicular phase to be approximately 45 mL/min (47), while the total uterine flow at term has been estimated to be as high as 750 mL/min (40), representing an almost 17-fold increase in flow to the uterus. Improvements in techniques to estimate blood flow in the gravid uterus have suggested that this last calculation may be too high. Thaler et al.(43) used a transvaginal duplex Doppler ultrasonography system to compare the blood flow characteristics in the ascending uterine artery before and during pregnancy in the same patient and determined that there was a 3.5-fold increase in blood flow—still a significant increase in total blood flow to the gravid uterus.
REFERENCES
|
1. Silver M, Barnes RJ, Comline RS, Burton GJ: Placental blood flow: some fetal and maternal cardiovascular adjustments during gestation. J Reprod Fertil Suppl 1982, 31:139-60
2. Poston L: The control of blood flow to the placenta. Exp Physiol 1997, 82:377-87
3. Kliman HJ: Trophoblast infiltration. Reproductive Medicine Reviews 1994, 3:137-57
4. Kliman HJ: Trophoblast to human placenta. Encyclopedia of Reproduction, vol 4. Edited by Knobil E, Neill JD. San Diego, Academic Press, 1999, pp 834-46
5. Kearns M, Lala PK: Life history of decidual cells: a review. Am J Reprod Immunol 1983, 3:78-82
6. Graham CH, Lala PK: Mechanisms of placental invasion of the uterus and their control. Biochem Cell Biol 1992, 70:867-74
7. Graham CH, Lysiak JJ, McCrae KR, Lala PK: Localization of transforming growth factor-beta at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod 1992, 46:561-72
8. Clark DA: Cytokines, decidua, and early pregnancy. Oxf Rev Reprod Biol 1993, 15:83-111
9. Roth I, Fisher SJ: IL-10 is an autocrine inhibitor of human placental cytotrophoblast MMP-9 production and invasion. Dev Biol 1999, 205:194-204
10. Tabibzadeh S, Lessey B, Satyaswaroop PG: Temporal and site-specific expression of transforming growth factor-beta4 in human endometrium. Mol Hum Reprod 1998, 4:595-602
11. Kliman HJ: Trophoblast infiltration. Reproductive Medicine Reviews 1994, 3:137-57
12. Feinberg RF, Kao LC, Haimowitz JE, Queenan JT, Jr., Wun TC, Strauss JF, Kliman HJ: Plasminogen activator inhibitor types 1 and 2 in human trophoblasts. PAI-1 is an immunocytochemical marker of invading trophoblasts. Lab Invest 1989, 61:20-6
13. Graham CH, Lala PK: Mechanism of control of trophoblast invasion in situ. J Cell Physiol 1991, 148:228-34
14. Strickland S, Richards WG: Invasion of the trophoblasts. Cell 1992, 71:355-7
15. Hendrickson MR, Kempson RL: Surgical pathology of the uterine corpus. Major Probl Pathol 1980, 12:36-98
16. Tabanelli S, Tang B, Gurpide E: In vitro decidualization of human endometrial stromal cells. J Steroid Biochem Mol Biol 1992, 42:337-44
17. Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J: Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril 1994, 62:497-506
18. Lessey BA: The use of integrins for the assessment of uterine receptivity. Fertil Steril 1994, 61:812-4
19. Pijnenborg R, Bland JM, Robertson WB, Brosens I: Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 1983, 4:397-413
20. Zhou Y, Genbacev O, Damsky CH, Fisher SJ: Oxygen regulates human cytotrophoblast differentiation and invasion: implications for endovascular invasion in normal pregnancy and in pre-eclampsia. J Reprod Immunol 1998, 39:197-213
21. Huppertz B, Kertschanska S, Demir AY, Frank HG, Kaufmann P: Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res 1998, 291:133-48
22. Genbacev O, DiFederico E, McMaster M, Fisher SJ: Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum Reprod 1999, 14:59-66
23. Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I: The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta 1981, 2:303-16
24. Pijnenborg R, Robertson WB, Brosens I: Trophoblast invasion and formation of the basal plate in the human placenta. Bibl Anat 1982, 22:69-73
25. Robertson WB, Brosens I, Pijnenborg R, De Wolf F: The making of the placental bed. Eur J Obstet Gynecol Reprod Biol 1984, 18:255-66
26. Robertson WB, Brosens I, Landells WN: Abnormal placentation. Obstet Gynecol Annu 1985, 14:411-262
27. Lyall F, Bulmer JN, Kelly H, Duffie E, Robson SC: Human trophoblast invasion and spiral artery transformation: the role of nitric oxide. Am J Pathol 1999, 154:1105-14
28. Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, Fisher SJ: Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol 1997, 151:1809-18
29. Enders AC, King BF: Early stages of trophoblastic invasion of the maternal vascular system during implantation in the macaque and baboon. Am J Anat 1991, 192:329-46
30. Hustin J, Schaaps JP: Echographic and anatomic studies of the maternotrophoblastic border during the first trimester of pregnancy. Am J Obstet Gynecol 1987, 157:162-8
31. Rodesch F, Simon P, Donner C, Jauniaux E: Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 1992, 80:283-5
32. Coppens M, Loquet P, Kollen M, De Neubourg F, Buytaert P: Longitudinal evaluation of uteroplacental and umbilical blood flow changes in normal early pregnancy. Ultrasound Obstet Gynecol 1996, 7:114-21
33. Burton GJ, Jauniaux E, Watson AL: Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol 1999, 181:718-24
34. Arias-Stella J, Jr., Arias-Velasquez A, Arias-Stella J: Normal and abnormal mitoses in the atypical endometrial change associated with chorionic tissue effect. Am J Surg Pathol 1994, 18:694-701
35. Kurjak A, Kupesic S, Hafner T, Kos M, Kostovic-Knezevic L, Grbesa D: Conflicting data on intervillous circulation in early pregnancy. J Perinat Med 1997, 25:225-36
36. Valentin L, Sladkevicius P, Laurini R, Soderberg H, Marsal K: Uteroplacental and luteal circulation in normal first-trimester pregnancies: Doppler ultrasonographic and morphologic study. Am J Obstet Gynecol 1996, 174:768-75
37. Moll W: Invited commentary: absence of intervillous blood flow in the first trimester of human pregnancy. Placenta 1995, 16:333-4
38. Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ: Onset of maternal arterial bloodflow and placental oxidative stress; a possible factor in human early pregnancy failure. Am J Pathol 2000, 157:
39. Assali NS, Rauramo L, Petonen T: Measurement of uterine blood flow and uterine metabolism. VIII. Uterine and fetal blood flow and oxygen consumption in early human pregnancy. Am J Obstet Gynecol 1960, 79:86-98
40. Assali NS, Douglass RA, Jr., Baird WW, Nicholson DB, Suyemoto R: Measurement of uterine blood flow and uterine metabolism. IV. Results in normal pregnancy. Am J Obstet Gynecol 1953, 66:248-53
41. Romney SL, Metcalfe J, Reid DE, Burwell CS: Blood flow of the gravid uterus. Ann N Y Acad Sci 1958, 75:762-9
42. Maini CL, Rosati P, Galli G, Bellati U, Bonetti MG, Moneta E: Non-invasive radioisotopic evaluation of placental blood flow. Gynecol Obstet Invest 1985, 19:196-206
43. Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, Brandes JM: Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol 1990, 162:121-5
44. Ueland K: Maternal cardiovascular dynamics. VII. Intrapartum blood volume changes. Am J Obstet Gynecol 1976, 126:671-7
45. Metcalfe J, Ueland K: Maternal cardiovascular adjustments to pregnancy. Prog Cardiovasc Dis 1974, 16:33-74
46. Ueland K, Metcalfe J: Circulatory changes in pregnancy. Clin Obstet Gynecol 1975, 18:41-50
47. Ziegler WF, Bernstein I, Badger
G, Leavitt T, Cerrero ML: Regional hemodynamic adaptation during the menstrual
cycle. Obstet Gynecol 1999, 94:695-9