|
MATERNAL DRUG USAGE
AND FETAL TERATOGENESIS |
Teratology is the study of abnormal prenatal development. A teratogenic exposure is one that can cause an embryo or fetus to develop abnormally and affect prenatal development by either:
∑ altering gene expression
∑ programmed cell death (apoptosis)
∑ affecting cell migration or proliferation, histogenesis, synthesis or function of proteins or nucleic acids.
Some teratogenic exposures act directly on the embryo, whereas others act through intermediates produced by maternal metabolism.
The stage of pregnancy during which an exposure occurs is extremely important:
∑ The first 2 weeks after conception is sometimes referred to as the "all-or-none" period, because toxic exposures during this time usually kill the embryo or produce no permanent effect if the embryo survives (1). Toxic exposures are unlikely to cause malformations, because the cells of the conceptus are pluripotent at this stage i.e. cells that are killed by an exposure can be replaced by other cells. However, if too many cells are damaged or die, the embryo will not survive. However, some mutagenic treatments during the preimplantation period produce malformations in rodent experiments, so the "all-or-none" rule does not always hold (2).
∑ Organogenesis (18–60 days post conception in humans) is the time during which the embryo is most sensitive to many teratogenic exposures and when most structural anomalies are produced. The fetal period is marked by rapid growth and maturation as well as active cell growth, proliferation and migration, particularly in the central nervous system. Teratogenic exposures during this period may cause fetal growth retardation, death or central nervous system dysfunction that may not be apparent until later childhood.
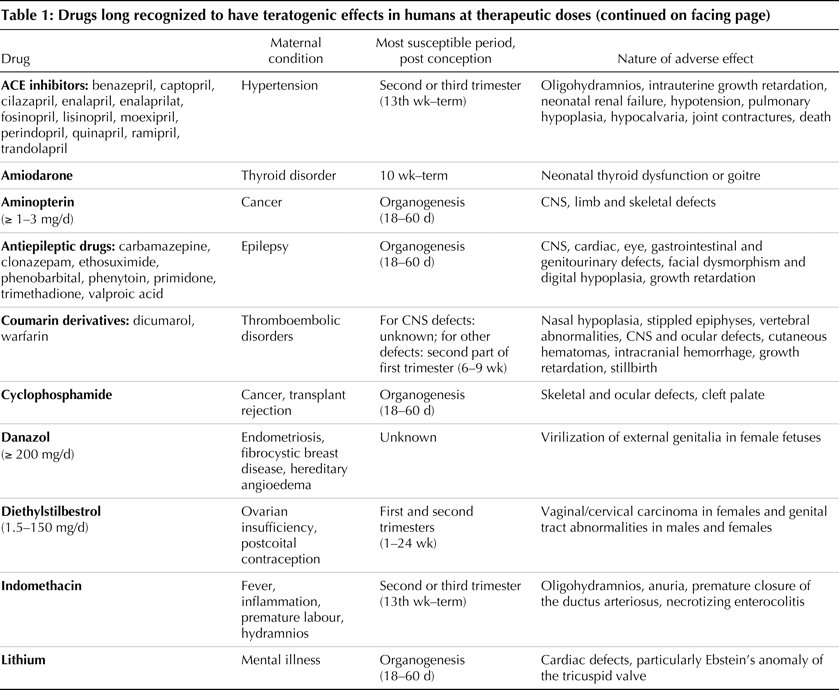
- Anticonvulsants (valproic acid, phenytoin, trimethadione) (1,2).
- Folic acid antagonists (methotrexate) (3).
- Anticoagulants (warfarin) (4).
- Fluorinated glucocorticoids (tiamcinolone) (5).
- Antineoplastic chemotherapeutic agents (6).
- Alcohol abuse - virtually all fetuses with fetal alcohol syndrome will be symmetrically growth retarded (may be severe) (7).
- Retinoic acid embryopathy.
- Lithium – In the 1970’s an international registry of 225 children who were exposed to lithium in utero reported an incidence of CHD that included six children (2.7%) with Ebstein’s anomaly (a four-hundred fold higher value than the unexposed population) (8,9). This was a biased study and more recent epidemiological studies suggest that the risk of CHD is actually much lower tan originally reported. In a prospective cohort study of 148 women, no significance in the rate of CHD was proved for mothers taking lithium versus the control population (0.9% versus 0.8%). Ebstein’s anomaly was the cardiac malformation in the lithium treated pregnant women (10,11). Case control studies in fetuses with Ebstein’s anomaly and animal studies have however failed to demonstrate teratogenicity of lithium in therapeutic doses (12).
- Non-steroidal anti-inflammatory drugs – they are reversible, competitive inhibitors of prostaglandin synthesis, and can alleviate prostaglandin-induced uterine contractions. An important side effect is premature constriction of the ductus arteriosus, which can lead to congestive cardiac failure and hydrops in the fetus, or pulmonary hypertension in the neonate. Prenatal exposure to indomethicin has been associated with the development of persistent pulmonary hypertension in the neonate(13). Ductus closure in the fetus may lead to increased right ventricular afterload, tricuspid regurgitation, right-to-left shunting, and rarely congestive heart failure. On the discovery of ductal constriction in utero, the drug should be withdrawn, and immediate delivery if patency is not restored. Although non steroidals can cross the placenta, several studies have failed to demonstrate teratogenicity (14).
- Beta –mimetics. They are the second most common tocolytic agents used in the USA (15). Examples include ritrodine and terbutaline. They easily cross the placenta and have been associated with significant fetal cardiovascular complications, fetal hyperglycemia, hyperinsulinemia and intraventricular hemorrhage (16,17).
Fetal arrhythmias have been reported in association with beta- mimetrics (18).
Agents known to cause small for gestational age perinates:
- Heroin (may be as high as 70% and as high as 30% in mothers using methadone) (19,20).
- Cocaine (30% of cases) (21).
Agents suspected (but not proven) to increase the risk of IUGR:
- Heavy metals.
- Organic solvents.
- Insecticides.
- Anesthetic gas.
- Caffeine (in huge doses).
- Organic solvents.
- Gasoline sniffing.
- Heavy cannabis abuse.
Mercury and fish consumption
Some predatory fish accumulate particularly high levels of mercury that can be toxic, particularly to developing fetuses (22). Recent case reports of toxic exposure (23) and research suggesting that groups at risk may be unaware of past advisories (24) reinforce the need to highlight limiting the intake of contaminated species (25).
The toxin:
∑ Elemental mercury from rocks and soil exists naturally in background levels in lakes and streams but is concentrated in the environment by emissions from hydroelectric projects, the burning of garbage and fossil fuels, and industrial pulp and paper and mining processes (24).
∑ Microorganisms in lake and stream sediments convert elemental mercury to organic methylmercury, which binds tightly to the proteins in fish tissue and is concentrated in fish higher up the food chain.
∑ When ingested by humans, methylmercury is easily absorbed and retained by the body; it has a half-life in blood of about 44 days, which makes blood tests useful measures of acute exposure (26).
∑ It concentrates in new hair, and consecutive hair segments indicate a person's exposure history (26).
∑ Methylmercury is eliminated fecally as inorganic mercury (27).
∑ Methylmercury is a potent neurotoxin, causing axonal demyeliniation (28). Adults can experience symptoms months after an acute exposure consisting of ataxia, blurred vision, hearing deficits and paraesthesias (7). Fetuses are particularly sensitive to methylmercury, as shown by the more than 1400 infants from the Minimata area of Japan who were acutely exposed in utero when their mothers ate fish contaminated by a factory discharge. The children, often normal at birth, developed abnormal reflexes, problems with suckling and swallowing, gait, speech, and mental retardation (24). The effects of chronic, low-level exposure, typical of many Aboriginal populations in Canada (29) is less clear. There is no effective treatment for methylmercury exposure.
Health Canada judges 0.5 parts per million (ppm) to be the limit for total mercury content in commercial fish (22,25) The consumption of mussels, pollock, salmon, scallops, shrimp and sole — the majority of aquatic species consumed in Canada — are not of concern. Fish with a total mercury content between 0.5 and 1.5 ppm include fresh and frozen tuna (but not canned tuna, which consists of smaller, shorter-lived species with lower mercury levels), swordfish and shark (22,31).
Mercury levels in freshwater fish varies, but in general bass, pike, muskellunge and walleye have high levels and should be eaten in moderation (30).
REFERENCES |
- Zackai EH, Mellmann WJ, Neiderer B et.al. J Pediatr 1975;87:280.
- Hanson JW, Myrianthopoulos NC, Harvey MAS et.al. Risk of offspring of women treated with hydantoin anticonvulsants with emphasis on the fetal hydantoin syndrome. J Pediatr 1976;89:662.
- Milunsky A, Graef JW, Gaynor P. Methotrexate induced congenital malformations with a review of the literature. J Pediatr 1968;72:790.
- Hall JQ, Parti RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. Am J Med 1980;68:122.
- Katz VL, Thorp JM, Bowes WA. Severe symmetric growth retardation associated with topical use of triamcinolone. Am J Obstet Gynecol 1991;162:396.
- Krepart GV, Lotocki RJ. Chemotherapy during pregnancy. In: Allen H, Nisker JA (eds). Cancer in Pregnancy. New-York: Futura 1986.
- Sokol RJ, Miller SI, Reed G. Alcohol abuse in pregnancy: an epidemiological study. Alcohol Clin Exo Res 1980;4:135.
- Weinstein MR. The International Register of Lithium Babies. Drug Information Journal 1976;10:94.
- Weinstein MR, Goldfield MD. Cardiovascular malformations with lithium use during pregnancy. Am J Psychol 1975;132:529.
- Ferner RE, Smith LE. Lithium in pregnancy. Lancet 1992;339:869.
- Jacobsen SJ, Jones K, Johnson X et.al. Prospective multicenter study of pregnancy outcome after lithium exposure during first trimester. Lancet 1992;339:530.
- Leonard A, Hantson P, Gerber GB. Mutagenecity, carcinogenecity, and teratogenicity of lithium compounds. Mut Res 1995;339:131.
- Moise K. Effect of advancing gestational age on the frequency of fetal ductal constriction in association with maternal indomethacin use. Am J Obstet Gynecol 1993;168:1350.
- Norton ME. Teratogen update: Fetal effects of indomethacin administration during pregnancy. Teratology 1997;56:282.
- Katz VL, Farmer RM. Controversies in tocolytic therapy. Clin Obstet Gynecol 1999;42:802.
- Friedman DM, Blackstone J, Young BK et.al. Fetal cardiac effects of oral ritrodine tocolysis. Am J Perinatol 1994;11:109.
- Washington CH. Risks and complications of tocolysis. Clin Obstet Gynecol 1995;38:725.
- Hermanson MC, Johnson GL. Neonatal supraventricular tachycardia following prolonged maternal ritrodine administration. Am J Obstet Gynecol 1984;149:798.
- Naeye BL, Blanc W, Leblanc W et.al. Fetal complications of maternal heroin addiction: Abnormal growth, infections and episodes of stress. J Pediatr 1973;83:1055.
- Newman RG, Bashkow S, Calco D. Results of 313 consecutive live births of infants delivered to patients in the New York City methadone maintenance program. Am J Obstet Gynecol 1975;121:233.
- Fulroth R, Phillips B, Durand DJ. Perinatal outcome of infants exposed to cocaine and/or heroin in utero. Am J Dis CHILD 1989;143:905.
- Food safety facts on mercury and fish consumption [fact sheet]. Ottawa: Canadian Food Inspection Agency; May 2002.
- Schmer J. Mercury in seafood [letter]. CMAJ 2002;167(2):122,124.
- Abelson A, Gibson BL, Sanborn MD, Weir E. Identifying and managing adverse environmental health effects: 5. Persistent organic pollutants. CMAJ 2002;166(12):1549-54.
- Advisory: Information on mercury levels in fish. Ottawa: Health Canada; 2002 May 29.
- Ruedy J. Methylmercury poisoning [letter]. CMAJ 2001;165(9):1193-4.
- Weir E. Methylmercury poisoning [letter]. CMAJ 2001;165(9):1194.
- Weir E. Methylmercury exposure: fishing for answers. CMAJ 2001;165(2):205-6.
- Dumont C, Girard M, Bellavance F, NoŽl F. Mercury levels in the Cree population of James Bay, Quebec, from 1988 to 1993/94. CMAJ 1998;158(11):1439-45.
- Guide to eating Ontario sport fish, 2001–2002. 21st ed rev. Toronto: Ontario Ministry of the Environment; 2001.
- Wooltorton E. Facts om mercury and fish consumption. CMAJ 2002;167(8): 897.